Exercise
question and answers
question and answers

1) Which
of the following statements is not a correct statement about the trends when
going from left to right across the periods of periodic Table?
of the following statements is not a correct statement about the trends when
going from left to right across the periods of periodic Table?
(a) The
elements become less metallic in nature.
elements become less metallic in nature.
(b) The
number of valence electrons increases.
number of valence electrons increases.
(c) The
atoms lose their electrons more easily.
atoms lose their electrons more easily.
(d) The
oxides become more acidic.
oxides become more acidic.
ANSWER: – The atoms lose their electrons more easily.
2) Element
X forms a chloride with the formula XCl2, which is a solid with a high melting
point. X would most likely be in the same group of the Periodic Table as
X forms a chloride with the formula XCl2, which is a solid with a high melting
point. X would most likely be in the same group of the Periodic Table as
(a) Na
(b) Mg (c) Al (d) Si
(b) Mg (c) Al (d) Si
ANSWER:-b)
Mg
Mg
3) Which
element has
element has
(a) two
shells, both of which are completely filled with electrons?
shells, both of which are completely filled with electrons?
(b) the
electronic configuration 2, 8, 2?
electronic configuration 2, 8, 2?
(c) a
total of three shells, with four electrons in its valence shell?
total of three shells, with four electrons in its valence shell?
(d) a
total of two shells, with three electrons in its valence shell?
total of two shells, with three electrons in its valence shell?
(e)
twice as many electrons in its second shell as in its first shell?
twice as many electrons in its second shell as in its first shell?
ANSWER:-
(a) Neon
has two shells, both of which are completely filled with electrons (2 electrons
in K shell and 8 electrons in L shell).
has two shells, both of which are completely filled with electrons (2 electrons
in K shell and 8 electrons in L shell).
(b)
Magnesium has the electronic configuration 2, 8, 2.
Magnesium has the electronic configuration 2, 8, 2.
(c)
Silicon has a total of three shells, with four electrons in its valence shell
(2 electrons in K shell, 8
electrons in L shell and 4 electrons in M shell).
Silicon has a total of three shells, with four electrons in its valence shell
(2 electrons in K shell, 8
electrons in L shell and 4 electrons in M shell).
(d) Boron
has a total of two shells, with three electrons in its valence shell (2
electrons in K shell and 3 electrons in L shell).
has a total of two shells, with three electrons in its valence shell (2
electrons in K shell and 3 electrons in L shell).
(e)
Carbon has twice as many electrons in its second shell as in its first shell
Carbon has twice as many electrons in its second shell as in its first shell
4) (a)
What property do all elements in the same column of the Periodic Table as boron
have in common?
What property do all elements in the same column of the Periodic Table as boron
have in common?
(b)
What property do all elements in the same column of the Periodic Table as
fluorine have in common?
What property do all elements in the same column of the Periodic Table as
fluorine have in common?
ANSWER:-
a) All the elements in the
same column as boron have the same number of valence electrons (3).
same column as boron have the same number of valence electrons (3).
b) All the elements in the
same column as fluorine have the same number of valence electrons (7). Hence,
they all have valency equal to 1.
same column as fluorine have the same number of valence electrons (7). Hence,
they all have valency equal to 1.
5) An
atom has electronic configuration 2, 8, 7.
atom has electronic configuration 2, 8, 7.
(a)
What is the atomic number of this element?
What is the atomic number of this element?
(b) To
which of the following elements would it be chemically similar?
(Atomic numbers are given in parentheses.)
which of the following elements would it be chemically similar?
(Atomic numbers are given in parentheses.)
N (7)
F(9) P(15) Ar (18)
F(9) P(15) Ar (18)
ANSWER:-
(a) The
atomic number of this element is 17.
atomic number of this element is 17.
(b) It
would be chemically similar to F(9) with configuration as 2, 7.
would be chemically similar to F(9) with configuration as 2, 7.
6) The
position of three elements A, B and C in the Periodic Table are shown below −
position of three elements A, B and C in the Periodic Table are shown below −
Group
16 Group 17
16 Group 17
− …………. −
− …………. A
− …………. −
B …………. C
(a)
State whether A is a metal or non-metal.
State whether A is a metal or non-metal.
(b)
State whether C is more reactive or less reactive than A.
State whether C is more reactive or less reactive than A.
(c)
Will C be larger or smaller in size than B?
Will C be larger or smaller in size than B?
(d)
Which type of ion, cation or anion, will be formed by element A?
Which type of ion, cation or anion, will be formed by element A?
ANSWER:-
(a) A is a non-metal.
(b) C is less reactive than A, as
reactivity decreases down the group in halogens.
reactivity decreases down the group in halogens.
(c) C will be smaller in size than
B as moving across a period, the nuclear charge increases and therefore,
electrons come closer to the nucleus.
B as moving across a period, the nuclear charge increases and therefore,
electrons come closer to the nucleus.
(d) A will form an anion as it
accepts an electron to complete its octet.
accepts an electron to complete its octet.
7) Nitrogen
(atomic number 7) and phosphorus (atomic number15) belong to group 15 of the
Periodic Table. Write the electronic configuration of
these two elements. Which of these will be more electronegative? Why?
(atomic number 7) and phosphorus (atomic number15) belong to group 15 of the
Periodic Table. Write the electronic configuration of
these two elements. Which of these will be more electronegative? Why?
ANSWER:-Nitrogen
….. 2… 5
….. 2… 5
Phosphorus.
2… 8… 5
2… 8… 5
Nitrogen
is more electronegative than phosphorus. As we move down in a group the number
of shells gradually increases therefore valence electrons move away from the
nucleus and the effective nuclear charge decreases. This causes the decrease in the tendency to attract
electron and hence electro
negativity decreases.
is more electronegative than phosphorus. As we move down in a group the number
of shells gradually increases therefore valence electrons move away from the
nucleus and the effective nuclear charge decreases. This causes the decrease in the tendency to attract
electron and hence electro
negativity decreases.
8) How
does the electronic configuration of an atom relate to its position in the
Modern Periodic Table?
does the electronic configuration of an atom relate to its position in the
Modern Periodic Table?
ANSWER:-In
the modern periodic table, atoms with similar electronic configurations are
placed in the same column.
the modern periodic table, atoms with similar electronic configurations are
placed in the same column.
9) In
the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements
with atomic numbers 12, 19, 21, and 38. Which of these have physical
and chemical properties resembling calcium?
the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements
with atomic numbers 12, 19, 21, and 38. Which of these have physical
and chemical properties resembling calcium?
ANSWER:-The
element with atomic number 12 has same chemical properties as that of calcium.
element with atomic number 12 has same chemical properties as that of calcium.
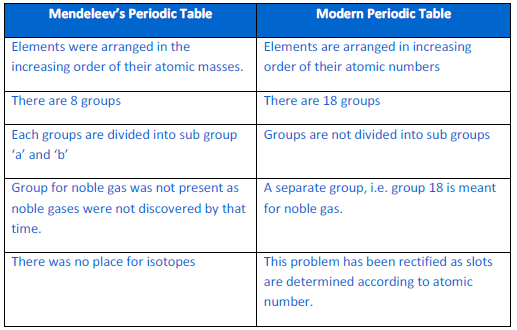
10) Compare
and contrast the arrangement of elements in Mendeleev’s
periodic Table and the Modern Periodic Table
and contrast the arrangement of elements in Mendeleev’s
periodic Table and the Modern Periodic Table
ANSWER:-